posters 5th Asia-Pacific NMR Symposium 2013
Solid State NMR Structural Studies of Duloxetine Hydrochloride (#134)
Duloxetine hydrochloride (pictured below) is an antidepressant currently finding widespread therapeutic use by working on pathways for serotonin and noradrenaline re-uptake inhibition, amongst other actions. Despite the importance of this new pharmaceutical, there is relatively little understanding of its structural diversity in the solid state, information which is vital for developing patent applications, formulation and manufacturing processes.
Our group initiated a structural survey of duloxetine hydrochloride, as well as its racemic form, for the purpose of understanding polymorphism in pharmaceuticals. Crystallisation from different solvents has not, as yet, revealed new polymorphs, but it has allowed us to fully characterise both the pharmaceutical grade (S)-isomer and the (RS)-racemate, as well as the (S)-acetone adduct.
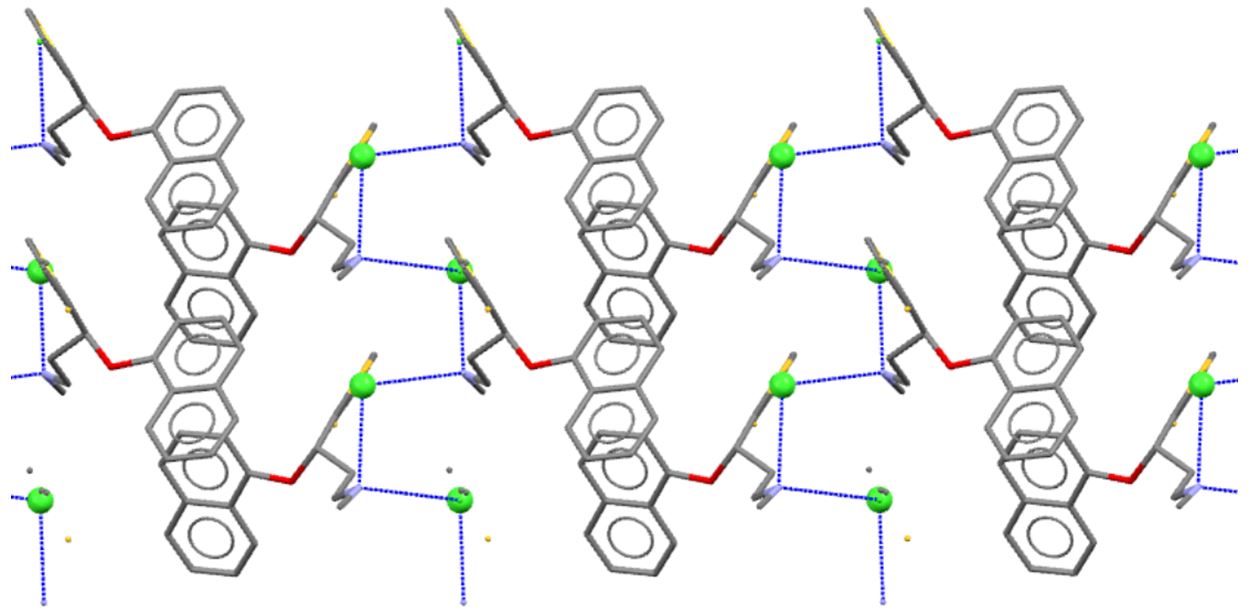
This presentation highlights the power of multi-nuclear 13C, 15N, and 35Cl solid state NMR techniques for probing the different structural motifs found in (S)-duloxetine and its isomers, as well as unambiguously determining the presence of the monoclinic form in commercial samples.
- M. Bhadbhade, J. Hook, C. Marjo, A. Rich and Q. Lin, Acta Cryst. (2009) E65, o2294.
- M. Bhadbhade, J. Hook, C. Marjo, A. Rich, Molecular Pharmaceutics (2011) 8, 2454.